The Cl Cl Bond Is Best Described as ___________
2 pts BONUS Questions 1 pt. When lead II nitrate is mixed with sodium chloride in aqueous solution what is the solid product.

A P Lecture Ch 8 9 11 Flashcards Quizlet
Chloride ion Cl- CID 312 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards.

. Polar covalent bond d. The ccl bond is best described as a nonpolar covalent. The forming of the H Cl bond releases energy.
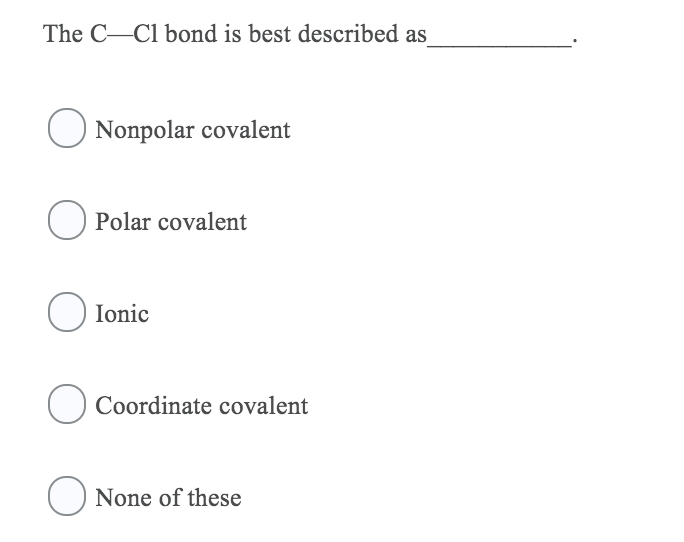
The C-C bond of chlorine is best described as Nonpolar covalent O Polar covalent lonic O Coordinate covalent ONone of these Expert Solution. The CCl bond is best described as___________. Course Title CHEM 2201.
Which of the following statements describes the transfer of heat in this system. The best description of why NH₄ forms an ionic bond with Cl is that its positive charge is attracted to the negative charge of Cl. Which best describes why nh4 can form an ionic bond with cl.
What best describes the Cl-Cl bond. What type of chemical bond holds the atoms together within a water molecule. 4 A bond is broken and energy is absorbed.
End question 2 the clcl bond of chlorine is best. Its outermost shell gains one or more electrons from cl. H2 Cl 2 2HCl Which statement best describes the energy change as bonds are formed and broken in this reaction.
The breaking of the Cl Cl bond releases energy. C the attraction that holds the atoms together in a polyatomic ion. End QUESTION 2 The ClCl bond of chlorine is best described how A Nonpolar.
Nonpolar covalent bond b. The C-Cl bond is best described as Nonpolar covalent Polar covalent Ionic O Coordinate covalent O None of these Identify the relationship between the following two structures. D the attraction between 2 nonmetal atoms.
The Cl Cl bond is best described as A nonpolar covalent B polar covalent C ionic from CHM 241 at Northern Virginia Community College. One that contains the maximum amount of solute that can be dissolved. It has a nitrogen atom that is strongly attracted to cl.
In a covalent compound the bond length can be defined as the distance between any two pairs of electrons. 50 the c cl bond is best described as a nonpolar. 2 A bond is broken and energy is released.
It has a negative charge that is spread over the entire ion. Classify the O-H bond in CH3OH as ionic polar covalent or nonpolar covalent. A Nonpolar covalent B Polar covalent C Ionic D Coordinate covalent E None of theseAns.
The Cl-Cl bond of Chlorine is best described how. 3 A bond is formed and energy is released. How is the C-O bond described.
Its positive charge is attracted to the negative charge of cl. The C-Cl bond is best described as A. Orbitals that are equivalent in energy are referred to as.
E the attraction between 2 metal atoms. As a result the seemingly metal ion is attracted to the negative non-metal ion. Up to 24 cash back Which of the following statements best describes the energy change as bonds are formed and broken in this reaction.
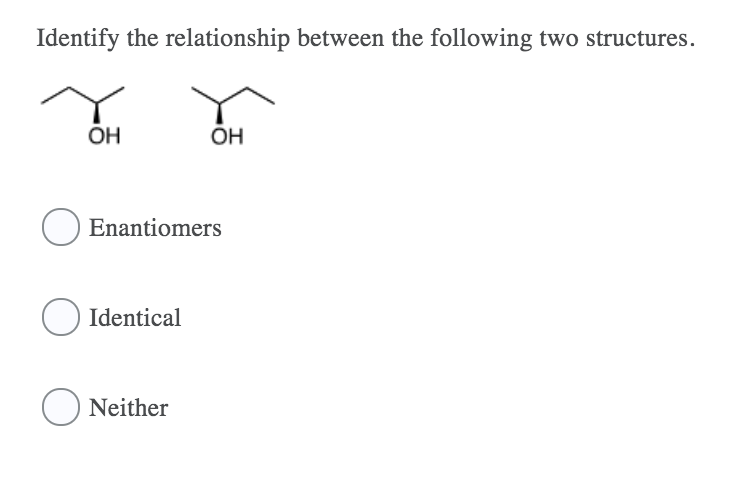
The breaking of the H H bond releases energy. OH OH Enantiomers Identical Neither Which of the following is the correct structure for the compound R-2-pentanol. An ionic bond forms as a result of transfer of electrons.
Each 33 Given the reaction. Induction and Polar Covalent bond Section. The Cl-Cl bond of chlorine is best described as Transcribed Image Text.
B c D The forming of the H Cl bond absorbs energy. Chloride is 1 3Cl 2 H 4 9 A covalent bond forms when 1 two nuclei share electrons in order to achieve a complete octet of electrons 2 atoms form ions and then electrostatic forces of attraction bond the ions together 3 repulsive forces between atoms are greater than the attractive forces. A Nonpolar covalent B Polar covalent C Ionic D Coordinate covalent E None of these Ans.
We have textbook solutions for you. A The forming of the H-Cl bond releases energy. The electron configuration of a carbon atom has how many electrons unpaired.
What is a saturated solution. ОН ОН ОН В. Pages 32 This preview shows page 4 - 14 out of 32 pages.
The C Cl bond is best described as___________. School University of New Haven. The electrostatic force of attraction between these ions is the.
1 An ionic bond is best described as A the sharing of electrons. The distance between two nuclei when the attraction is greatest. B Polar covalent.
How many distinct p orbitals exist in the 2nd electron shell. Which type of bond is. The distance between two nuclei when the repulsion is greatest.
B the transfer of electrons from one atom to another. 32 Explain in terms of electronegativity why an H-F bond is expected to be more polar than an H-I bond. The distance between the two largest atoms.
5 A 500-gram block of copper at 100C is carefully lowered into 1000 grams of water at 90C in an insulated container.
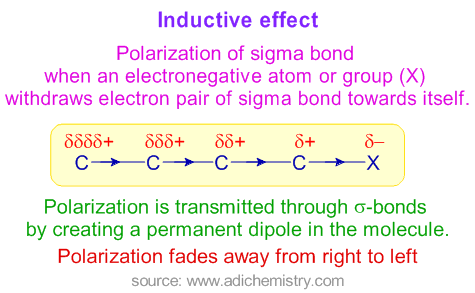
Inductive Effect Positive Negative Definition Examples Applications

Chemical Bonding Chunky Boards Ppt Video Online Download

Chem 1331 Exam 1 Study Material From Homeworks Flashcards Quizlet

Covalent Bonds Flashcards Quizlet
Solved The C Cl Bond Is Best Described As Nonpolar Covalent Chegg Com

1 St Semester Exam In High School Chemistry Examination In January Of Ppt Download

Covalent Bonding Molecular Compounds Multiple Choice Review
Solved The C Cl Bond Is Best Described As Nonpolar Covalent Chegg Com

A P Lecture Ch 8 9 11 Flashcards Quizlet

Biology Exam 1 Chapters 1 4 Flashcards Quizlet

Chapter 2 Basic Aspects Of Biochemistry Organic Chemistry Acid Base Chemistry Amino Acids Protein Structure And Function And Enzyme Kinetics Flashcards Quizlet
Solved The C Cl Bond Is Best Described As Nonpolar Covalent Chegg Com

Multiple Questions Multiple Choice Questions 1 A Carbon Hydrogen Bond In Ethane Ch3ch3 Is Best Described A A Highly Polar B Essentially Course Hero

Si Mock Exam 2 Exam Chem 1030 Fundamentals Chemistry I Studocu

Chemistry Chap 4 Flashcards Quizlet

Solved Your Answer Is Incorrect Try Again The Cl Ci Bond Of Chegg Com

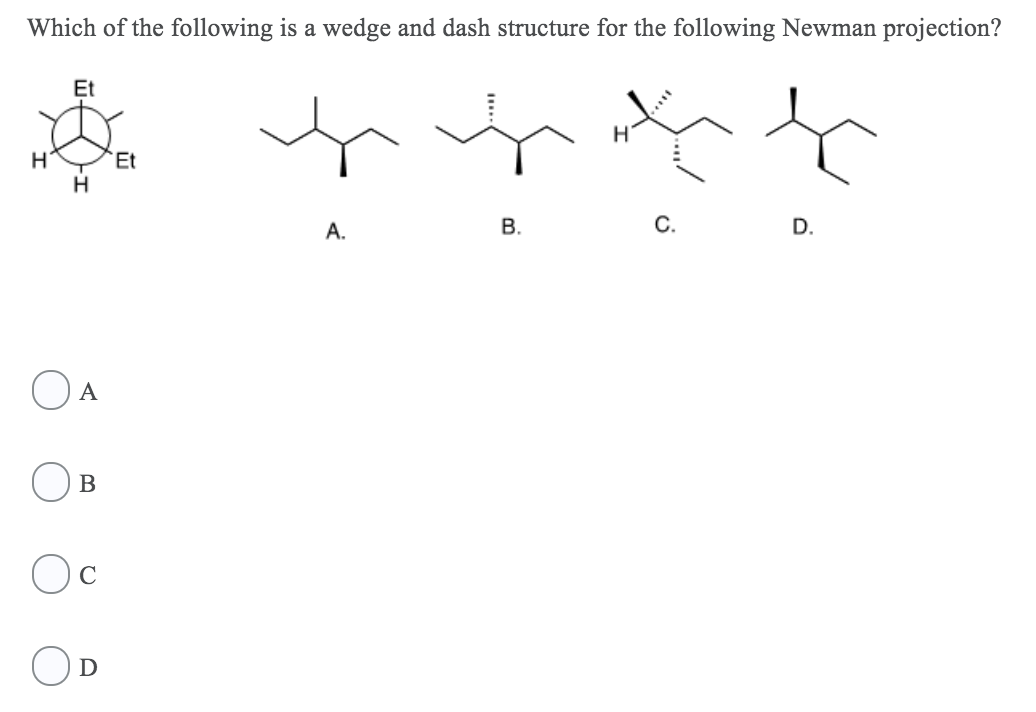
Organic Chemistry Chapter 6 Alkyl Halides Nucleophilic Substitution And Elimination Organic Studocu